Environmental gamma-ray spectrometry in 2000 words or less
Inspired by Venkatesh Rao’s 100-opinion brainstorm Twitter thread, I decided to put into words all my salient opinions about environmental gamma-ray spectrometry as a self-assesment exercise. The result is the following short piece. I wrote it in one sit, and I quite enjoyed the experience. This “technique” is an effective and fun way to fathom how much one truly knows about a subject. Bonus: it helps nail down potential points of improvement in your knowledge.
But what exactly is gamma-ray spectrometry?
The technique consists of measuring the quantity of gamma-ray emitting radionuclides in a sample, thanks to radiation’s interaction with matter. These rays can interact with certain materials (a scintillating crystal, a semi-conductor, etc.), producing electrical currents that are amplified and interpreted by a computer, and a histogram of pulses is generated. We radiometry specalists call it “gamma spectrum”.
Of all nuclear analytical methods, gamma-ray spectrometry’s got to be one of the most exciting because it can be non-destructive. This means that you can literally see through walls.
That’s only possible because gamma radiation is pervasive and can get through nearly everything, even concrete. It’s the first kind of radiation that’s measured in the wake of an accident justly because it’s everywhere and can be measured from a distance.
The necessary equipment to measure gamma radiation is nowhere fancy. Actually, you can do it at home with just a couple hundred of dollars, a sound board, and a permit to handle radioactive standards. Take a look at this sweet project by PhysicsOpenLab (figure below).
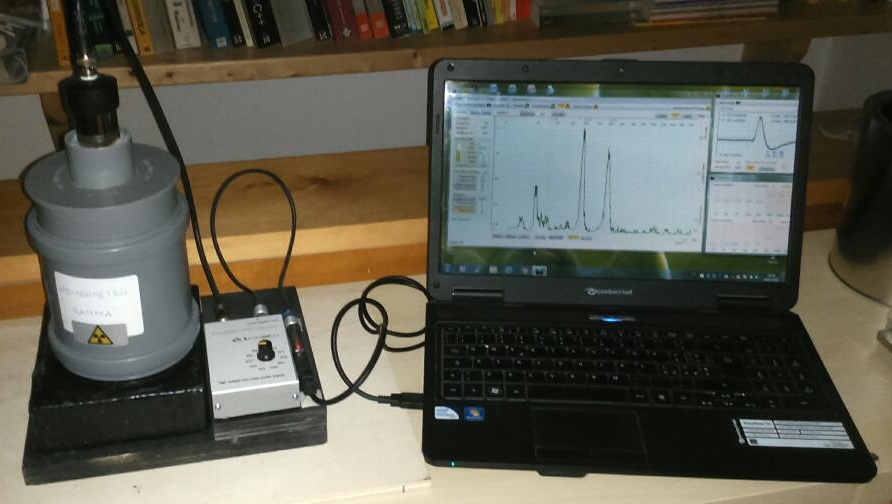
Figure 1: A home-made gamma-ray spectrometer. Source: PhysicsOpenLab
If you are not a research institute or you can’t have the authorities to issue a permit, then you can promptly replace the radioactive standard with something that naturally emits gamma radiation to calibrate your spectrometry system, like a handful of potassium salt.
You read it correctly. Potassium. Gamma rays aren’t originated from nuclear waste only.
It may turn out as a shock to some, but you, me, and every living thing emit gamma radiation, mostly because of all the potassium-40 in our bodies. To illustrate this point, a research paper from the Radioprotection and Dosimetry Institute (IRD) in Rio de Janeiro shows a marked increase in K-40 concentration on a group of subjects’s quadriceps after a short period of bulking. Sick peaks, bro.
But more on that later.
Knowing your system
“Why do I need a radioactive source to measure gamma radiation from stuff?” Because not every gamma-ray detector is born equal. They will have different shapes, dimensions, and impurities. Some can “see” a lot of the radiation that’s emitted nearby. Others not so much.
So you need a means to “calibrate” your system, which means assessing how your detector behaves when it interacts with photons along a large range of possible energies. This is called “detector’s efficiency”, and for gamma-ray detectors it usually looks like this:
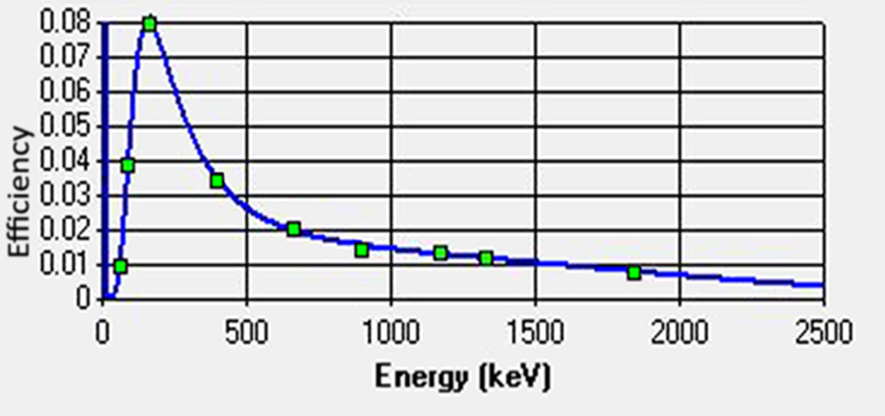
Figure 2: A typical efficiency curve of a HPGe detector.
If you’re asking what the heck is this curve, it’s a quadratic logarithmic function, and it’s different for every combination of detector plus sample shape. Who said radiometry specialists don’t have fun?
Once you have nailed the efficiency curve for your geometry (detector + sample shape), you can now acquire your spectrum and watch the peaks grow!!… Over a period of 3 days or more, depending on what you’re analyzing.
Unless you’re analyzing some real hot stuff, your samples will have very low activity to produce any good peaks (good = 10k counts or more).
A typical gamma-ray spectrum of a soil sample looks like this:
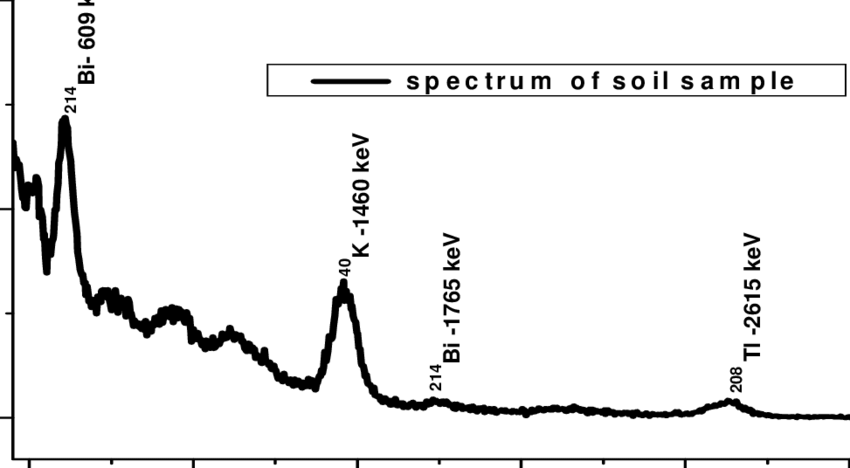
Take that beautiful potassium-40 peak at 1,46 MeV for instance. If I want to know how much K-40 is in this particular sample, all I have to do is calculate the area of the peak, take into account the detector efficiency for this energy, and the probability of a that gamma emission happening.
Presto! With all these informations and a spectrum at hand, we can measure the radionuclides concentration of everything on Earth. But if we are talking about naturally ocurring radionuclides in environmental samples, then there’s a catch. Actually, a LOT of catches.
Measuring naturally ocurring radioactive material (NORM)
Environmental samples usually comprise a bunch of measurable gamma-emitting radionuclides. Example: K-40, Be-7, and a good part of the U-238 and Th-232 decay chains. K-40 and Be-7 are well behaved and nice to measure. The U and Th decay chain, on the other hand, are their problematic cousins
Although very important, these nuclides don’t emit gamma rays in a particularly flattering region of the spectrum. Their emission is very feeble and in the low-energy region, which is both prone to efficiency calculation errors and interference with x-rays.
But good news! There are other nuclides in their decay chain that are excellent gamma emitters, like Ac-228, Bi-214, and so on.
For example, this is how the U-238 decay chain looks like.
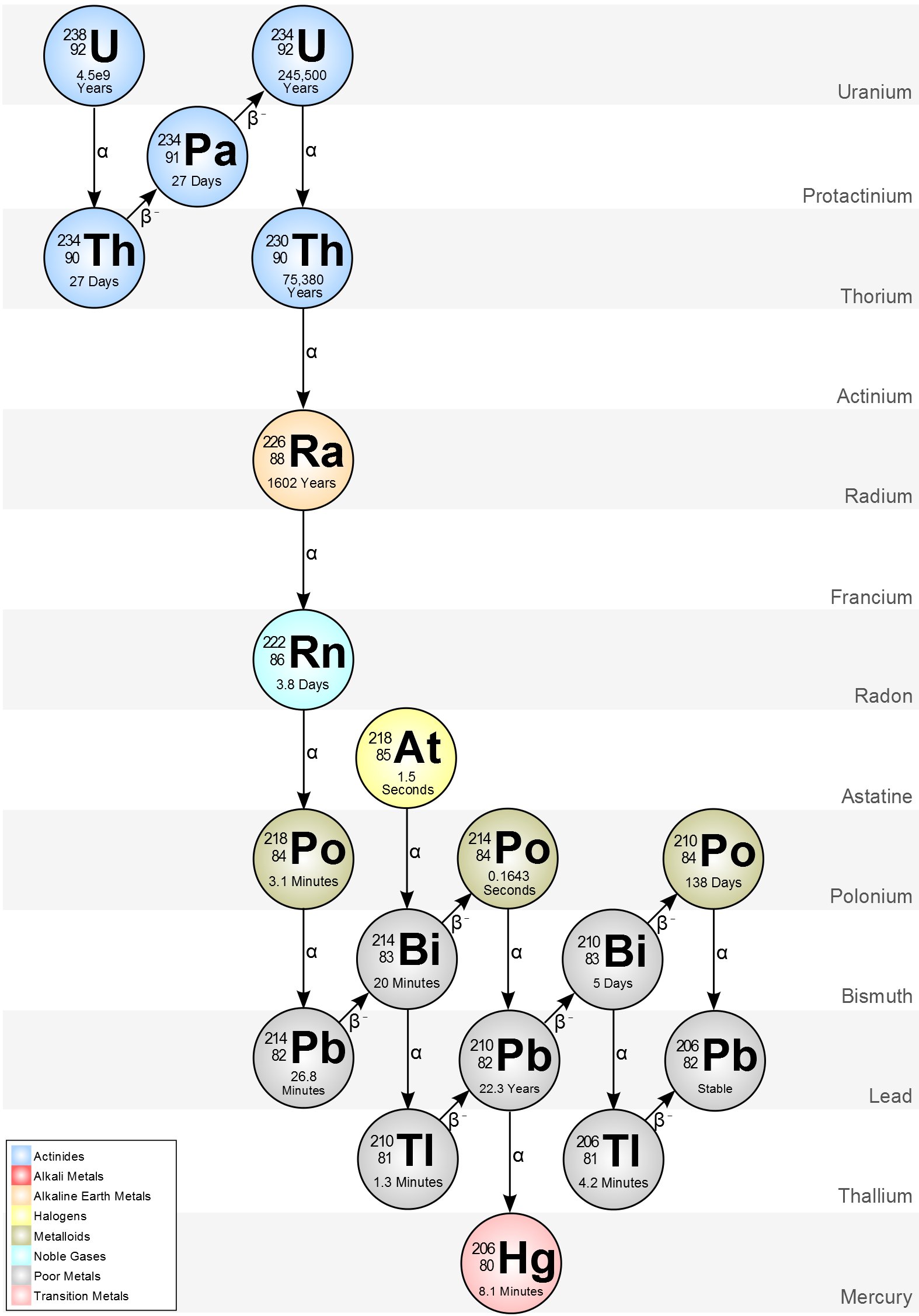
If we want to analyze something at the top of the chain (say, pure U-238), all we have to do is wait until it produces enough radioactive “daughters” and reach what we call secular equilibrium, which is when the whole chain has the same activity.
Secular equilibrium is reached after a few half-lives of the daughter radionuclide. If you want to know more about a samples U-238 activity for instance, all you have to do is wait 180 days and measure it indirectly through its daughter Pa-234m.
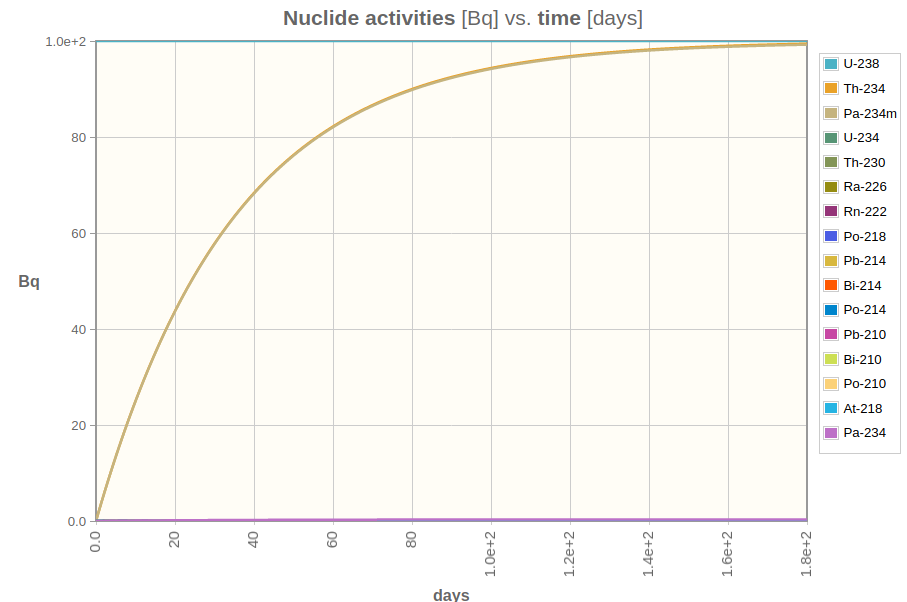
Figure 3: Pa-234m build-up curve in function of time. After a while, it equals to its parent nuclide’s activity.
Pa-234m is an excellent gamma emitting nuclide, but its emission probability is too small, so your sample must have a lot of uranium and you’ll wait a lot to measure it. That, my folks, is why gamma-ray spectrometry isn’t the best technique for uranium measurements.
The radon problem
Now back to more common, layperson gamma-ray measurements. Suppose we want to measure Ra-226 in water samples. This is a good gamma emitting radionuclide, but it happens to emit almost at the same energy as U-235. The alternative is to measure the next best thing in the chain, Bi-214.
Ra-226 is a natural radionuclide, and we’re here on Earth for billions of years, so the whole chain must have reached secular equilibrium already, right? No need to wait, right??
WRONG.
Between Ra-226 and its gamma emitting daughters there’s Rn-222, which is volatile. Yeah, your secular equilibrium has literally flown away during this time.
The solution is to store your sample in a sealed recipient for at least 30 days. If the radon can be imprisoned inside the recipient, the whole decay chain will eventually achieve secular equilibrium, right?
Yeah, good luck with that.
The problem with radon is that it’s a noble gas and it transpasses. Every. Effing. Material. High-density polyethilene won’t do. Glass won’t do. Acryllic sort of does. Metal does too, but it attenuates some of the radiation that should be hitting the detector.
Another catch: radon is everywhere. The largest part of all background radiation dose we are subject to comes from the radon in the air and which exudes from the soil, especially if your soil is composed of thorium-rich rocks and your house is badly ventilated.
This is particularly worrisome in countries where buildings have their windows and doors closed most of the time. The cheapest and easiest solution is ventilate the rooms from time to time. Building homes a few inches above ground also helps.
Even then, there will be traces of radon in the air, including the laboratory where your gamma spectrometry system is. There are labs that keep an inert atmosphere in their spectrometry rooms by pumping nitrogen in.
(Pro-tip: you can create a small inert atmosphere by diverting the evaporating liquid nitrogen from the dewars that refrigerate your gamma detector directly into its lead chamber. I call it “The Poor Man’s Monaco Laboratory”.)
Yet another catch when it comes to NORM nuclides: almost every nuclide is susceptible to coincidence summing. Unless your radioactive standard is made of exactly these nuclides and in the same geometry, you’ll have to apply corrections.
And that sucks.
More interference
It’s usually a good practice to wrap the inner side of your detector chamber with a tin + copper layer to attenuate x-rays from the lead chamber itself. Most commercial lead chambers already come with the extra protection.
But there are also a multitude of other sources of interference that still can hinder your low activity analysis, like a high enough baseline that may impact your MDA (minimum detectable activity).
One of the largest sources of background noise in gamma spectra is the decceleration (or Bremsstrahlung) of particles when they interact with the detector matter. Kinetic energy is transformed into something else: in this case, a headache to the technician.
That’s why you usually don’t want strong peaks at higher energies, lest it increases the background continuum at lower energy region of interest.
Simulating is hard
Obviously there are tools to circumvent problems like this. CERN, NIST, LANL, and many other institutes provide tools to either generate an efficiency curve for custom geometries, or ways to correct for coincidence summing, or both. There are some commercials tools as well.
But the art of generating efficiency curves for nuclear instruments (by the Monte Carlo method) depends on knowing exactly, down to micrometers, how your detector is designed, and this is not always trivial.
For instance, high-purity germanium detectors (HPGe) end up with a dead layer in their crystals which doesn’t respond to the gamma rays colliding on it, and you have to account for it if you want to make an accurate simulation.
Accurate Monte Carlo simulations are even tougher to get when you work with low energies. Above 200 KeV, you don’t have to worry much about what attenuators are made of. Just knowing their thickness will be a good enough approximation.
Below 200 keV, the interaction between radiation and matter is a function of a plethora of variables, including the average atomic number of the material.
Good luck simulating that.
Ideally, when dealing with low energies you should have a bespoke efficiency curve for each analysis. That’s easier if you have an XRF (x-ray fluorescence) machine, so you know exactly which elements compose your sample.
Summing up
Most of the NORM nuclides can be measured through peaks above 200 keV. Ra-226 measurements, for instance, are done by the 609 keV peak from Bi-214. But sometimes you want to avoid minor interferences and true coincidence summing corrections. In this case, analysis becomes trickier again because higher energy peaks, like that of Bi-212 at ~1.6 MeV has a low emission probability, and detectors have a poor efficiency around this energy range.
Given all constraints, long waiting times, and a whole lot of interference, environmental radiometry may wind up sounding a bit boring.
And it is.
But when you deal with nuclear measurements, boring is the new good.